Disclaimer:
The authors are solely responsible for the content of this report. Material included herein does not represent the opinion of the European Community, and the European Community is not responsible for any use that might be made of it.
Back to overview reports
Estuarine input of macronutrients that can be considered in estuaries (see earlier, fig. 1):
Transformation processes are described in further detail in the following section, ‘1.2.2 Sink and source function regulation’ , for each macronutrient separately, together with their potential input sources.
Output to the coastal waters is the cumulative result of estuarine input and estuarine processing, defining whether the estuary is a source or sink as a whole for the coastal zone. This defines the so-called filter capacity of an estuary (Soetaert et al. 2006, Quiel et al. 2011). The latter states which nutrient ratios are eventually held in the coastal zone, and consequently which phytoplankton communities will follow. This will define further flow through to the linear food chain and/or microbial loop; hence, very important for overall ecological functioning and provisioning of several ecosystem services. Excess of nutrients can cause eutrophication problems, often found to be detrimental for system functioning (Rousseaux et al. 2006; see also earlier, ‘1.1 Biogeochemical processing for N, P and Si in Northern temperate estuaries’ ). Once nutrient loads arrive in the sea they are no longer to be managed. Hence, estuarine management has a crucial role in ensuring the filter capacity for nutrients.
Following processes (Sanders et al. 1997, Statham et al. 2011) occur in both the water column and sediments:
Inputs for inorganic nitrogen species are associated with atmospheric exchange (N-deposition), surface water run-off, rivers and ground water input. Input by rivers is of primary importance. Either source can be direct, or indirect (through organic matter input). The importance of groundwater input is largely unknown.
Dissolved organic compounds are not considered, but are possibly of more importance than assumed at present (Statham et al. 2011).
Phosphate is very particle reactive. In estuaries a buffering mechanism exists at the suspended particulate matter-water and sediment-water interface.
In turbid estuaries (SPM > 50 mg/l), phosphate is strongly adsorbed to iron-oxyhydroxides (fig 3). As particulate phosphorus arrives in the high salinity zone, competition for adsorption sites by stronger anions (OH-, F-, SO42-, …), causes suspended matter to release phosphate to the water column again (van Beusekom & Brockmann 1998, Deborde et al. 2007, Statham et al. 2011).
An equilibrium would be reached for concentrations of 5 µM (0,015 mg P/l), and desorption is promoted at higher concentrations (Soetaert et al. 2006 and refs herein). Deborde et al. (2007) found adsorption and desorption processes of phosphate to be independent of phosphate concentrations. However, in the Gironde concentrations never exceeded 5 µM (Deborde et al. 2007). Paradoxically, large removal by suspended matter relative to sediment burial implies less storage of phosphorus within the estuary, since phosphate is easily released again in the high salinity zones (Jickels et al. 2000, van der Zee et al. 2007). This mechanism of phosphate release is observed in the conservative mixing diagram of many temperate estuaries, as so-called bell-shaped profiles (fig. 4). For very high suspended particulate matter concentrations as those observed in the Gironde (9000 mg/l), some phosphorus did not desorb towards the sea. This delayed desorption could be attributed to increased primary production in the high salinity reaches, were light became more available again. Most desorption was observed in the 0 to 12 PSU salinity range (Deborde et al. 2007). Also in the Scheldt and Elbe, most desorption was observed within this range, and from this point on (sal > 10 PSU) decreasing linearly towards the sea because of dilution (conservative behaviour) (van Beusekom & Brockmann 1998, van der Zee et al. 2007).
Organic matter mineralisation for dissolved inorganic phosphate is a rather slow process, and its importance is largely dependent on residence times (69 days for the Gironde). Nevertheless, for the Gironde example less than 10 % is mineralized in the sediment while about 50 % is mineralised in the estuarine turbidity maximum zone (Deborde et al. 2007). Most organic matter mineralisation appears to occur within the turbidity maximum zone, usually to be found in the lower salinity reaches (Abril et al. 1999). The higher mineralisation rate can be attributed to the larger reactive surface area of the suspended particulate matter and the availability of more degradable organic matter opposed to higher salinity reaches (van Beusekom & Brockmann 1998).
Furthermore the residence time (and thus time for biogeochemical transformation processes) of non-conservative behaving suspended matter is significantly longer than for conservative behaving dissolved substances (Statham et al. 2011).
The suspended particulate matter might also settle before it arrives in the high salinity zone and form a pool of phosphate buried in the sediment. The phosphate burial in the sediment is very dependent on the redox state. When the iron-oxyhydroxides in the sediments are reduced, phosphate is again released to the water column. Also, when sediments are exported more downstream, iron-sulfate complexes are formed preferential upon iron-phosphate complexes, and phosphate is released (Statham et al. 2011). Combined with re-suspension phosphate release can be further enhanced (van der Zee et al. 2007). However, some phosphate may be more permanently buried when precipitated as authigenic minerals like apatites (van Beusekom & Brockmann 1998).
In general, phosphorus retention is promoted by high oxygen concentrations, opposite to the requirements for nitrogen retention (Soetaert et al. 2006). Tidal mudflats studied in the Humber, showed very small and variable phosphate fluxes comparable to fluxes in other temperate estuaries (Mortimer et al. 1998 and refs herein). Fluxes by the sediment-water exchange can be enhanced by macro-faunal activity through bio-irrigation (Mortimer et al. 1998, Braeckman et al. 2010).
Rivers are the most principal pathways for phosphorus input. Atmospheric inputs can be considered negligible compared to atmospheric deposition for nitrogen. Groundwater inputs are largely unknown and can be function of bottom geo-morphology (e.g. if a so-called ‘iron curtain’ is present, groundwater input can be inhibited). Phosphate dynamics are difficult to generalize and are very dependent on the physico-chemical properties of the environment (Statham et al. 2011).
Phosphate and particulate inorganic phosphorus (adsorbed to SPM) often are the main phosphorus species involved in phosphate dynamics (van der Zee et al. 2007). However, also dissolved organic phosphorous appears to become of more interest in phosphorus dynamics, but remains largely unstudied up to now (Statham et al. 2011).
Back to top
How do TIDE estuaries function as a filter for nutrients?
What are the important factors controlling ecosystem functioning?
What is important in establishing a zonation for estuaries?
What parameters should be used to define and evaluate measure targets?
Which measures are suitable to improve the physical characteristics and chemical water quality?
Which variables limit estuarine primary production?
An interestuarine comparison for ecology in TIDE
Table of content
- 1. Introduction
- 1a. Biogeochemical processing for N, P and Si in Northern temperate estuaries
- 1b. Nutrient fluxes
- 1c. Primary production
- 1d. Differences and similarities between Elbe, Scheldt, Humber and Weser
- 2. Material and methods
- 2a. Study area & period
- 2b. Physics
- 2c. Biogeochemistry
- 3. Results
- 3a. Physics 2
- 3b. Biogeochemistry 2
- 4. Discussion
- 4a. Estuarine patterns
- 4b. Different sink and source functions for nutrients
- 4c. Oxygen deficiencies
- 4d. Primary production 2
- 5. Conclusion
- 5a. What are the important factors controlling ecosystem functioning within the TIDE estuaries?
- 5b. How do TIDE estuaries function as a filter for nutrients?
- 5c. How can we avoid oxygen deficiency situations in the TIDE estuaries?
- 5d. Which variables limit primary production in the TIDE estuaries?
- 5e. Lessons learnt from TIDE: towards ecosystem services and measures, recommendations for further estuarine management
- 6. References
Authors:
by Geerts L, Maris T, Meire P
With contributions of Beauchard O, Schöl A, Vandenbruwaene W., Van Damme S, Wolfstein K, Manson S, Saathoff S, Soetaert K, Cox T, Meire A, (February 2013)
by Geerts L, Maris T, Meire P
With contributions of Beauchard O, Schöl A, Vandenbruwaene W., Van Damme S, Wolfstein K, Manson S, Saathoff S, Soetaert K, Cox T, Meire A, (February 2013)
1b. Nutrient fluxes
Nutrient fluxes are considered for dissolved inorganic nitrogen, phosphate and dissolved silicate. These macronutrients are essential and key elements in limiting primary production in estuaries (if light is not limiting, see further ‘1.2.1 Hydro-morphology’ ) (Underwood & Kromkamp 1999, Carbonnel et al. 2009). Nutrient fluxes with respect to estuaries can be measured for (1) estuarine input, (2) transformation processes within the estuary and for (3) estuarine output to the coastal waters (Statham et al. 2011).Estuarine input of macronutrients that can be considered in estuaries (see earlier, fig. 1):
- Exchange at the atmosphere – water boundary;
- Coastal input;
- Input from the main tributaries and the upper boundary of the estuary;
- Ground water input.
Transformation processes are described in further detail in the following section, ‘1.2.2 Sink and source function regulation’ , for each macronutrient separately, together with their potential input sources.
Output to the coastal waters is the cumulative result of estuarine input and estuarine processing, defining whether the estuary is a source or sink as a whole for the coastal zone. This defines the so-called filter capacity of an estuary (Soetaert et al. 2006, Quiel et al. 2011). The latter states which nutrient ratios are eventually held in the coastal zone, and consequently which phytoplankton communities will follow. This will define further flow through to the linear food chain and/or microbial loop; hence, very important for overall ecological functioning and provisioning of several ecosystem services. Excess of nutrients can cause eutrophication problems, often found to be detrimental for system functioning (Rousseaux et al. 2006; see also earlier, ‘1.1 Biogeochemical processing for N, P and Si in Northern temperate estuaries’ ). Once nutrient loads arrive in the sea they are no longer to be managed. Hence, estuarine management has a crucial role in ensuring the filter capacity for nutrients.
Hydro-morphology
Nutrient fluxes are highly seasonal (reflected in hydrodynamics) and vary spatially (reflected in salinity and morphology) (Sanders et al. 1997, Statham et al. 2011).Salinity gradient
Due to tidal mixing and freshwater input, estuaries demonstrate a strong salinity gradient. Changes in ionic strength, dissolved organic matter content, pH and in the carbonate system between fresh and saltwater end members are induced, resulting in changed speciation of nutrient species and overall reactivity of the estuarine system (Statham et al. 2011).Residence time and freshwater discharge
Residence times may vary from several weeks to several months for various estuaries. Residence time also varies seasonally in function of freshwater discharge. Usually freshwater discharge is lower in summer, generating more extended residence times. A long residence time implies more opportunity for sediment-water exchange and water column biogeochemistry to occur (Statham et al. 2011). Furthermore, it allows algae to grow, rather than being flushed out of the estuary (Underwood & Kromkamp 1999).Suspended matter dynamics and light climate
Usually, high suspended particulate matter concentrations with a typical turbidity maximum zone near the oligohaline zone are observed in estuaries. This is due to river input, continuous re-suspension of the fine fraction of the sediment inventory and import from coastal waters. Non-conservative mixing makes that the suspended matter residence times are much longer than those observed for water and dissolved substances. For the Humber a suspended particulate matter residence time of 18 years was calculated (Turner 1990 in Statham et al. 2011). Suspended particulate matter increases the surface area for adsorption and desorption processes to occur and provides a hotspot for microbial and algal activity. However, suspended matter concentrations may also modify the light climate and hence, by consequence rather inhibit primary production by algae, than stimulate it (Statham et al. 2011).Intertidal and subtidal area
Intertidal and subtidal area provides a large sediment surface at which exchange with the water column can take place. The larger this area, the more nutrients can be exchanged (Statham et al. 2011; for more details, see further, ‘1.2.2 Sink and source function regulation’ per macronutrient).Sink and source function regulation
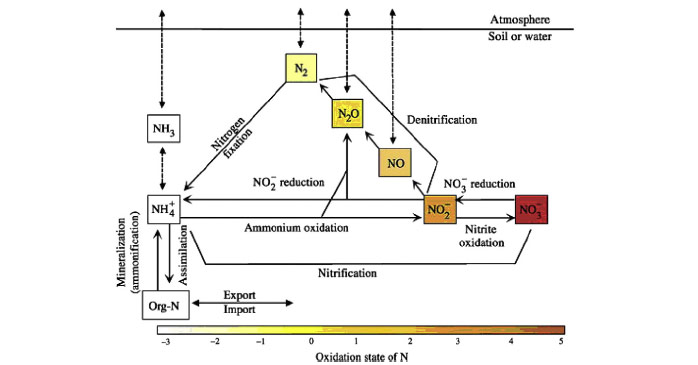
Nitrogen
Inter-conversions (fig. 2) occur over a large range of redox states (-3 to +5). However, reactive nitrogen is the most important to consider, since this is most involved in biological activity. Therefore, most studies indeed focussed upon dissolved and mostly inorganic nitrogen species, more specifically upon nitrate. Most present estuaries are well oxygenated, and nitrate is thus most abundant. Also nitrate is mostly related to eutrophication issues. Gaseous forms (NOx, N2O) are much less studied. Nonetheless, these could give us more valuable information on nitrification and denitrification rates (Statham et al. 2011).Following processes (Sanders et al. 1997, Statham et al. 2011) occur in both the water column and sediments:
- export to adjacent coastal oceans;
- biological uptake, might be in resistant forms within the sediment;
- losses to the atmosphere by nitrification and denitrification processes;
- organic matter mineralisation.
Inputs for inorganic nitrogen species are associated with atmospheric exchange (N-deposition), surface water run-off, rivers and ground water input. Input by rivers is of primary importance. Either source can be direct, or indirect (through organic matter input). The importance of groundwater input is largely unknown.
Dissolved organic compounds are not considered, but are possibly of more importance than assumed at present (Statham et al. 2011).
Phosphorus
Phosphorus is regulated by inorganic and biological interactions. Hence, phosphorus dynamics are dependent on the presence of different particulate and dissolved phosphorus species and biological activity. Phosphorus is required for photosynthesis, general metabolism, cell wall synthesis and energy transfer (e.g. ATP) (Statham et al. 2011). Phosphate and particulate inorganic phosphorus bound to suspended matter both seem to be the most abundant phosphorus species involved in phosphorus dynamics (van der Zee et al. 2007).Phosphate is very particle reactive. In estuaries a buffering mechanism exists at the suspended particulate matter-water and sediment-water interface.
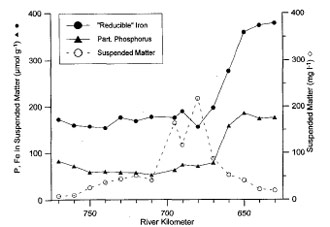
In turbid estuaries (SPM > 50 mg/l), phosphate is strongly adsorbed to iron-oxyhydroxides (fig 3). As particulate phosphorus arrives in the high salinity zone, competition for adsorption sites by stronger anions (OH-, F-, SO42-, …), causes suspended matter to release phosphate to the water column again (van Beusekom & Brockmann 1998, Deborde et al. 2007, Statham et al. 2011).
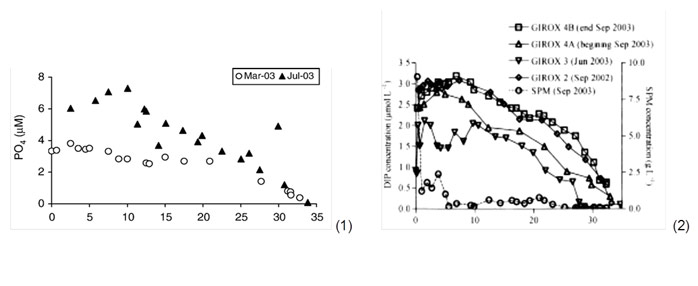
An equilibrium would be reached for concentrations of 5 µM (0,015 mg P/l), and desorption is promoted at higher concentrations (Soetaert et al. 2006 and refs herein). Deborde et al. (2007) found adsorption and desorption processes of phosphate to be independent of phosphate concentrations. However, in the Gironde concentrations never exceeded 5 µM (Deborde et al. 2007). Paradoxically, large removal by suspended matter relative to sediment burial implies less storage of phosphorus within the estuary, since phosphate is easily released again in the high salinity zones (Jickels et al. 2000, van der Zee et al. 2007). This mechanism of phosphate release is observed in the conservative mixing diagram of many temperate estuaries, as so-called bell-shaped profiles (fig. 4). For very high suspended particulate matter concentrations as those observed in the Gironde (9000 mg/l), some phosphorus did not desorb towards the sea. This delayed desorption could be attributed to increased primary production in the high salinity reaches, were light became more available again. Most desorption was observed in the 0 to 12 PSU salinity range (Deborde et al. 2007). Also in the Scheldt and Elbe, most desorption was observed within this range, and from this point on (sal > 10 PSU) decreasing linearly towards the sea because of dilution (conservative behaviour) (van Beusekom & Brockmann 1998, van der Zee et al. 2007).
Organic matter mineralisation for dissolved inorganic phosphate is a rather slow process, and its importance is largely dependent on residence times (69 days for the Gironde). Nevertheless, for the Gironde example less than 10 % is mineralized in the sediment while about 50 % is mineralised in the estuarine turbidity maximum zone (Deborde et al. 2007). Most organic matter mineralisation appears to occur within the turbidity maximum zone, usually to be found in the lower salinity reaches (Abril et al. 1999). The higher mineralisation rate can be attributed to the larger reactive surface area of the suspended particulate matter and the availability of more degradable organic matter opposed to higher salinity reaches (van Beusekom & Brockmann 1998).
Furthermore the residence time (and thus time for biogeochemical transformation processes) of non-conservative behaving suspended matter is significantly longer than for conservative behaving dissolved substances (Statham et al. 2011).
The suspended particulate matter might also settle before it arrives in the high salinity zone and form a pool of phosphate buried in the sediment. The phosphate burial in the sediment is very dependent on the redox state. When the iron-oxyhydroxides in the sediments are reduced, phosphate is again released to the water column. Also, when sediments are exported more downstream, iron-sulfate complexes are formed preferential upon iron-phosphate complexes, and phosphate is released (Statham et al. 2011). Combined with re-suspension phosphate release can be further enhanced (van der Zee et al. 2007). However, some phosphate may be more permanently buried when precipitated as authigenic minerals like apatites (van Beusekom & Brockmann 1998).
In general, phosphorus retention is promoted by high oxygen concentrations, opposite to the requirements for nitrogen retention (Soetaert et al. 2006). Tidal mudflats studied in the Humber, showed very small and variable phosphate fluxes comparable to fluxes in other temperate estuaries (Mortimer et al. 1998 and refs herein). Fluxes by the sediment-water exchange can be enhanced by macro-faunal activity through bio-irrigation (Mortimer et al. 1998, Braeckman et al. 2010).
Rivers are the most principal pathways for phosphorus input. Atmospheric inputs can be considered negligible compared to atmospheric deposition for nitrogen. Groundwater inputs are largely unknown and can be function of bottom geo-morphology (e.g. if a so-called ‘iron curtain’ is present, groundwater input can be inhibited). Phosphate dynamics are difficult to generalize and are very dependent on the physico-chemical properties of the environment (Statham et al. 2011).
Phosphate and particulate inorganic phosphorus (adsorbed to SPM) often are the main phosphorus species involved in phosphate dynamics (van der Zee et al. 2007). However, also dissolved organic phosphorous appears to become of more interest in phosphorus dynamics, but remains largely unstudied up to now (Statham et al. 2011).
Silica
Dissolved silica is mainly regulated by biology along the estuarine gradient. Thus fluxes are highly seasonal dependent. It is a critical component for diatoms to build up their cell walls (frustules). Hence, in summer lowest dissolved silica concentrations and corresponding highest biogenic silica (opal) concentrations are observed. The residence time has to be sufficiently long for the diatoms to take up the dissolved silica. Highest concentrations are observed in winter and behave conservatively along the mixing gradient. Diatoms have been shown to take up 50 to 70 % of the total silica load during the productive period (Carbonnel et al. 2009). When algal growth is inhibited, or stopped (dissolved silica limitation < 0.01 mM ~ 0.3 mg DSi/l, light, grazing), diatom frustules might sink and be buried in the sediment. Biogenic silica dissolution increases with salinity and some bacterial communities have been shown to increase dissolution too. Furthermore, phytoliths within macrophytes (e.g. Reed) can be added by topsoil erosion or directly from vegetation of riverbanks or tidal marshes to the biogenic silica pool. Tidal marshes are most likely very important silica recyclers to the estuary. Retention of biogenic silica in tidal marshes, originating from imported biogenic silica of diatoms via suspended matter sedimentation and phytoliths in Reed, combined with increased residence times and increased sediment-water interactions, intensifies biogenic silica dissolution processes. Tidal marshes have been demonstrated to be a source for dissolved silica when freshwater discharge and dissolved silica concentrations within the estuary are low (Struyf et al. 2006, 2007). Recycling capacity has been shown to be higher for young marshes than for older, more elevated marshes. This can be related to different sedimentation rates (Struyf et al. 2007). In intertidal mudflats silica fluxes were found very small within the Humber estuary. However, this might not be as representative, since primary production within this estuary is very low. However, low dissolution patterns could also be linked to rather low overlying dissolved silica concentrations (Mortimer et al. 1998). Fluxes by the sediment-water exchange are in general enhanced by macro-faunal activity through bio-irrigation (Mortimer et al. 1998, Braeckman et al. 2010). However, most dissolved silica originates from rock weathering and can be associated with riverine input (Carbonnel et al. 2009, Statham et al. 2011). In rivers dissolved silica concentrations usually remained high all year round and biogenic silica low (Carbonnel et al. 2009), except sometimes in larger rivers (e.g. Elbe, personal communication Andreas Schöl 2013). In the tidal river dissolved silica concentrations are almost completely consumed, while biogenic silica concentrations rise. Contribution of phytoliths in the Scheldt to detrimental biogenic silica was found non-significant. Hence, delivery of silica from Reed phytoliths could not account for the increase in biogenic silica during the productive period. In summer dissolved silica concentrations can be consumed up to limitation levels (Van Damme et al. 2005, Soetaert et al. 2006). Within the estuary biogenic silica is mainly delivered by diatoms, especially in summer. In winter, most is imported as dissolved silica (Carbonnel et al. 2009). When silica export is inhibited, nuisance algal blooms can appear downstream in the coastal zone (Rousseaux et al. 2006). Opposed to the summer bloom, the spring bloom had no significant effect upon silica dynamics (Carbonnel et al. 2009). To understand regulating mechanisms, it is important to also consider biogenic silica fluxes (Carbonnel et al. 2009). Dynamics in the freshwater zone drive the dynamics in the coastal zone (Rousseaux et al. 2006).Nutrient ratios
The ideal nutrient ratio for diatom growth is defined by Redfield as 106/16/1/16 corresponding to C/N/P/Si respectively (Billen & Garnier 2007). When light climate and residence time are suitable, primary production will increase with nutrient concentrations rising. However, within most estuaries light is limiting (SPM > 10 mg/l, mixing depth) and change in nutrient ratios will only affect algal growth there where a better light climate is reached, e.g. in the estuarine plume of the Humber estuary, beyond Spurn point (Nedwell et al. 2002, Soetaert et al. 2006). Last decades, nutrient loads have increased, because of population increase and land use change. Combined with unequal water treatment efficiency, this has lead to disproportional nutrient ratios in many estuaries (Statham et al. 2011). Nutrient ratios define phytoplankton species composition and succession along the estuarine gradient; hence define ecological functioning (Carbonnel et al. 2009). Phosphorus is typically limiting in freshwater, while nitrogen is limiting in estuarine and coastal systems. However, large riverine nitrogen input and more efficient phosphorus removal have shifted the limitation in many northern temperate estuaries to a limitation for phosphorus (Sanders et al. 1997, Soetaert et al. 2006). Nitrogen is mostly related to diffuse input sources and therefore much more difficult to manage. Although to a lesser extent, also silica loads have been altered by human activities. Hydrological measures such as embankments have reduced the contact with the riparian vegetation and consequently lowered silica input in the estuary. Furthermore, deforestation has lowered the baseflow delivery of silica twice to threefold in temperate European watersheds (Struyf et al. 2010). Hence, lowered silica input and increasing nitrogen and phosphorus loads have increased overall silica limitation in estuaries.
1a. Biogeochemical processing for N, P and Si in Northern temperate estuaries
1c. Primary production
Important to know
Reports / Measures / Tools
| Report: | Zonation of the TIDE estuaries (Functioning) |
|---|---|
| Report: | Interestuarine comparison: Hydro-geomorphology (Functioning) |
Management issues
How can we avoid oxygen deficiency situations?How do TIDE estuaries function as a filter for nutrients?
What are the important factors controlling ecosystem functioning?
What is important in establishing a zonation for estuaries?
What parameters should be used to define and evaluate measure targets?
Which measures are suitable to improve the physical characteristics and chemical water quality?
Which variables limit estuarine primary production?